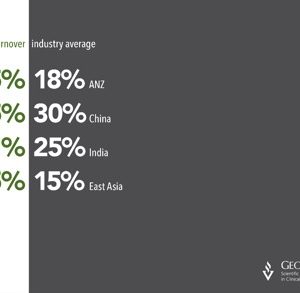
Outsourcing criteria within the Asia-Pacific
George Clinical (GC) conducted a brief survey on sponsors who are currently not a customer of GC. 30 companies were surveyed by GC’s Business Development Managers across six countries which included the United States, China, Australia, Korea, United Kingdom and Israel. The primary objective of the survey was to determine the services and countries that were important […]
Read More