Emerald Clinical delivers India arm of this landmark trial with on-time recruitment and 100% retention.
Situation
“Nearly half of all people with type 2 diabetes will develop chronic kidney disease (CKD), causing a high risk of kidney failure and cardiovascular disease and impacting their quality and length of life, even with the current best available care. This huge unmet need is why it was so important for us to initiate the landmark CREDENCE renal outcomes trial,” says Vlado Perkovic, CREDENCE Steering Committee co-chair, Professor of Medicine, University of New South Wales. CREDENCE was the first dedicated renal outcomes trial in patients with CKD and type 2 diabetes on the background of standard of care, and evaluated the efficacy and safety of canagliflozin versus placebo in preventing clinically important renal and cardiovascular outcomes in patients. This study heralds the first new drug therapy in more than 15 years for slowing down the progression of CKD in patients with type 2 diabetes, which could be transformational for these patients worldwide.
The trial enrolled approximately 4,400 patients in 34 countries including 144 patients in India, where Emerald Clinical was engaged to execute trial operations. Emerald Clinical was uniquely positioned for this task due to the deep experience of our India operational teams as well as the world-renowned scientific leaders in nephrology associated with Emerald Clinical. In addition, Emerald Clinical’s parent organization, The George Institute, has a more than a fifteen-year presence in India where, under the leadership of Vivekanand Jha, Executive Director of The George Institute for Global Health, India, and President of the International Society of Nephrology, they have been conducting outstanding nephrology research.
Challenges
India was brought into the trial later than other countries to further enrich the recruitment pool. Given the fact that more than 62 million people in India have diabetes and that 17% of the population has some form of CKD, India was a natural destination to broaden the study’s recruitment. However, due to the late addition of the country, it was critical for the success of the study to have Indian sites on board and to recruit in a short time frame. Quick assimilation into partner CRO’s systems and to the existing global network of a highly complex trial with many partners was also required.
Key Results
Clear and consistent communication pathways needed to be established among all stakeholders. Consideration of possible differences in renal clinical practices in India and knowledge of cultural behaviors was also essential to assure clear communication and consistent results.
Solutions
Emerald Clinical’s awareness of the value of including India in clinical research and our deep experience and long presence in India allowed us to fast-track India’s operations from recruitment to site initiation and management of the trial. Our familiarity with regulatory issues, clinical practice and cultural aspects positioned us uniquely to organize, lead and monitor the study efficiently.
Emerald Clinical’s involvement ensured adequate dedicated and trained resources on the study, and our existing operational teams in India were able to maximize recruitment efforts and ramp up rapidly. Our nimbleness, flexibility and ability to adapt to the sponsor and partner CRO’s systems helped to make recruitment successful.
Our team also developed a schematic as part of the project plan for communicating with the sponsor and other CROs that ensured complete understanding of the appropriate access requirements. All communication gaps were eliminated or reduced through continuous discussion with the study groups, the sponsor and the CRO.
We also provided close monitoring and intervention through a National Leader, and our scientific leadership model ensured that all aspects of the India operations would remain clearly focused on the intended scientific purpose of the trial.
Results
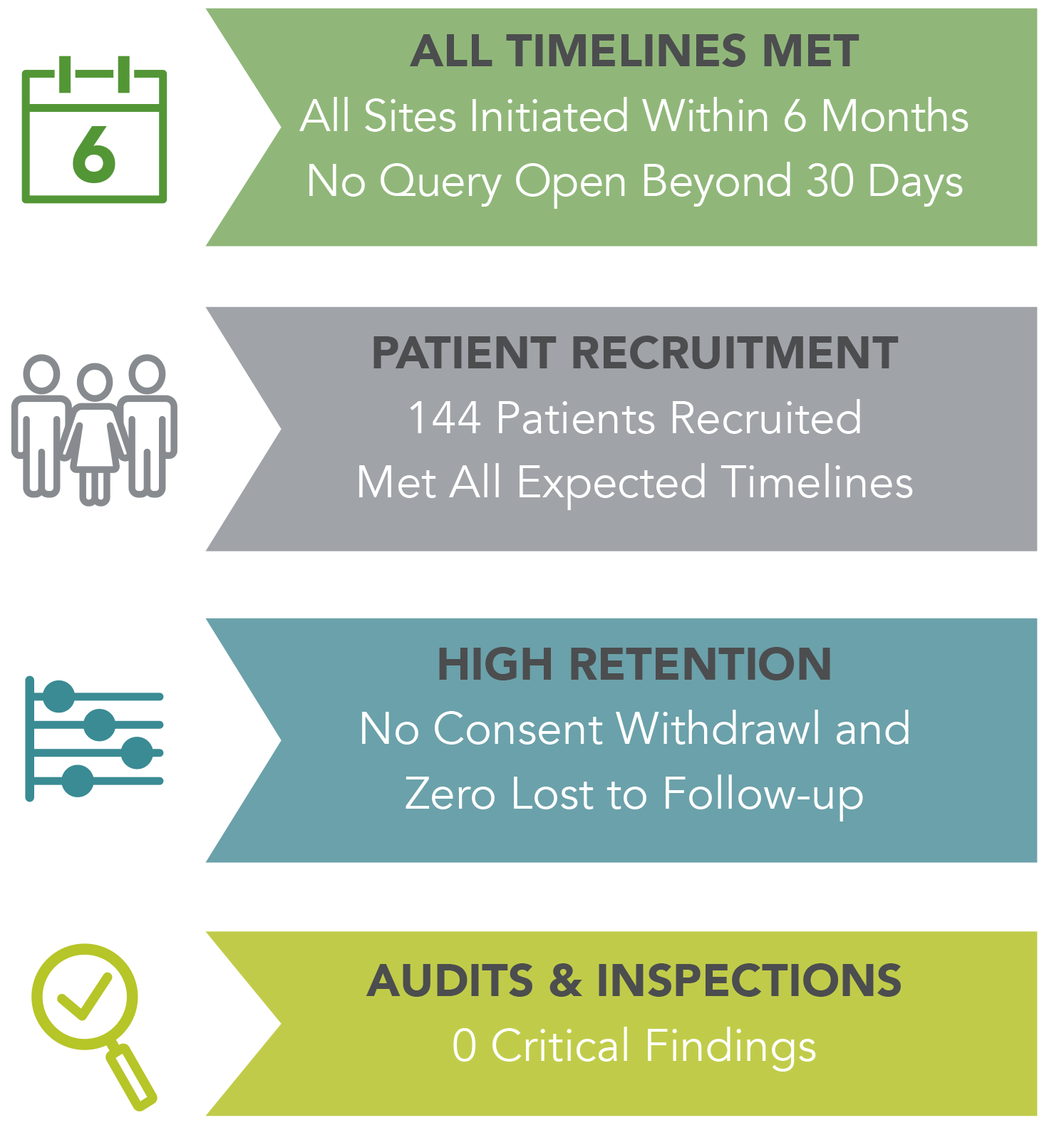
All sites were initiated within six months from regulatory approval. Patient recruitment met all expected timelines. In nine months, 144 patients were recruited for the trial. Retention was very high, with no consent withdrawal and zero lost to follow up. As this was an end-point study, retention was critical. All data entry and query resolution timelines met expectations and no query was open beyond 30 days. There were no critical findings from various sponsor oversight visits, audits and regulatory inspections.
At Emerald Clinical we are excited that patients from India were able to participate in this important trial. We have great confidence in our operational teams in India and understand the value of doing renal research there. “India possesses the ideal combination of the right skill sets and testing environments to generate innovative and disruptive models of healthcare delivery and evaluate their scalability and affordability in real life situations. Such models can be replicated with some modifications in countries with similar healthcare challenges,” says Vivekanand Jha, Executive Director of The George Institute for Global Health, India, and President of the International Society of Nephrology.
1 Phase III CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) clinical trial evaluating the efficacy and safety of INVOKANA® (canagliflozin) versus placebo when used in addition to standard of care for patients with chronic kidney disease (CKD) and type 2 diabetes.
